Long-term 10-year outcomes study1
Objective
This study examined the long-term (>10 years) safety and efficacy of Enterra Therapy in patients with intractable nausea and vomiting.
Design
Retrospective, single-centre French study examining 50 consecutive patients with refractory chronic nausea and/or vomiting lasting at least 12 months (failure of 2 prokinetics and antiemetics) implanted with gastric electrical stimulator.

Primary endpoint
- Gastrointestinal Quality of Life Index (GIQLI) score.
- Primary outcome was reduction in nausea and/or vomiting using a 5-point score.
- Reduction in nausea and/or vomiting by 1 point, defined as >50% reduction in frequency.
Results
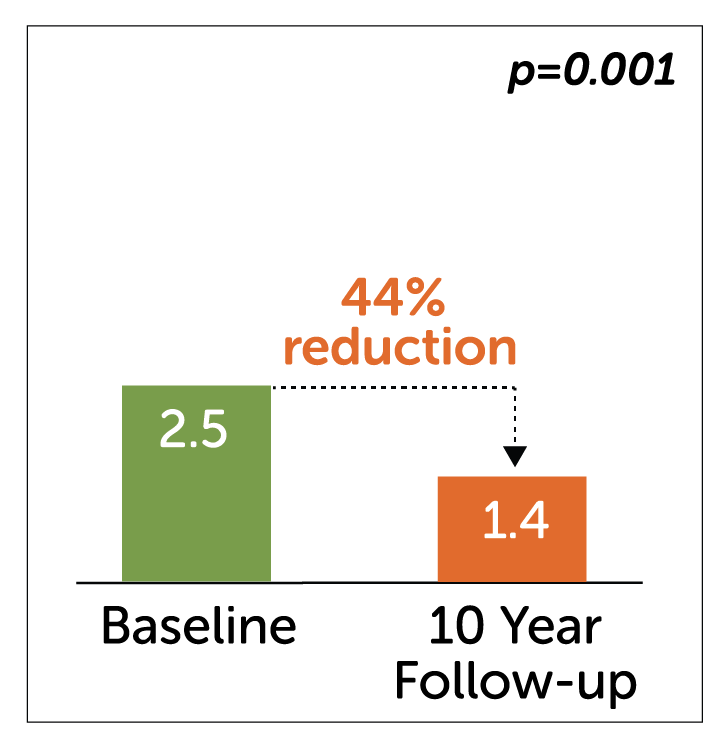
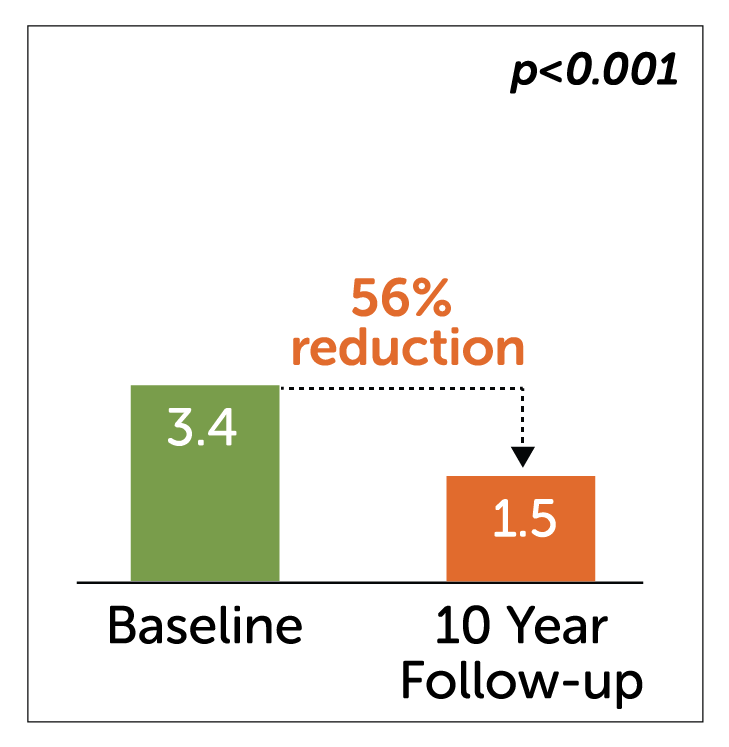
- In a retrospective study of 50 patients, Enterra Therapy significantly improved primary endpoint of nausea and vomiting in 54% of patients by greater than 50% at 10 years.
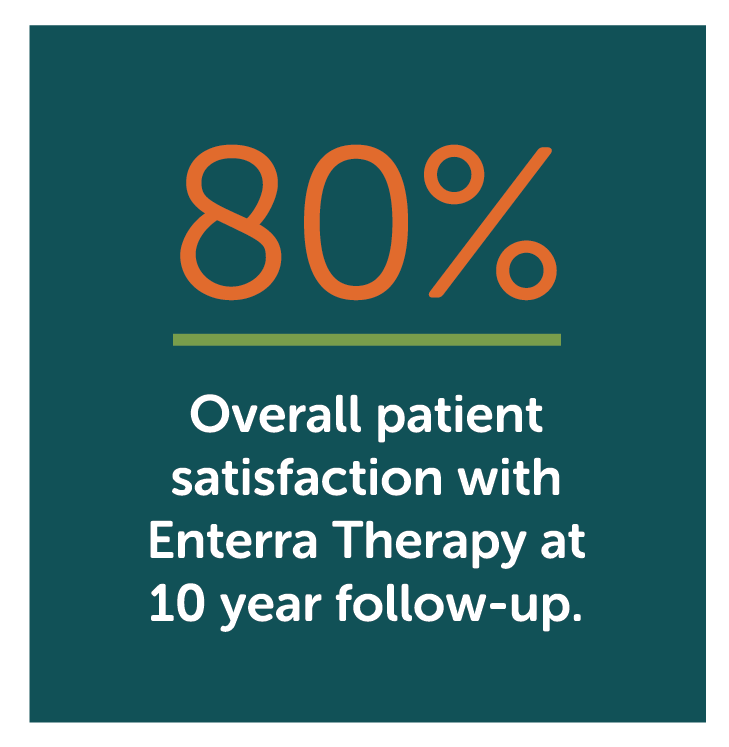
- Enterra Therapy significantly improved primary endpoint of Gastrointestinal QOL Index (GIQLI) score and patients reported high level of satisfaction (80%) with the therapy at 10 years.
Nausea
Vomiting
Gastrointestinal quality of life index (GIQLI) score
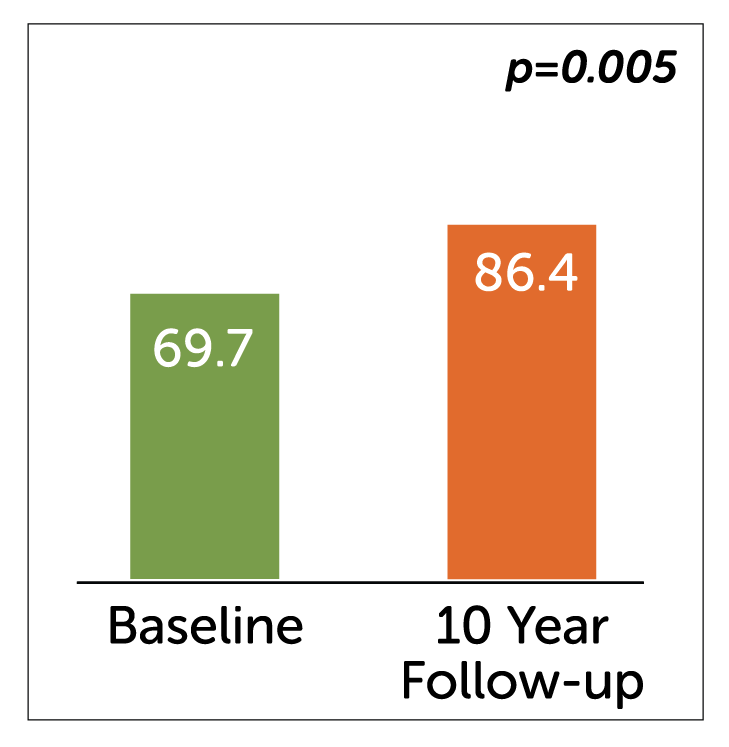
Median overall GIQLI patient satisfaction score
- Hedjoudje A, Huet E, Leroi AM, Desprez C, Melchior C, Gourcerol G. Efficacy of gastric electrical stimulation in intractable nausea and vomiting at 10 years: A retrospective analysis of prospectively collected data. Neurogastroenterol Motil. 2020;32(11):e13949. doi:10.1111/nmo.13949.
