Washington University 5-year Study on Enterra Therapy (2024)
Study Objective
Examining clinical outcomes of Enterra Therapy in the treatment of patients with chronic, intractable (Refactrory to 2x Drug Clases extending past 6 months) gastroparesis
Study Design
Prospective data from large (n=157), tertiary academic, single-center, 1 & 5 year follow-up on Enterra Therapy
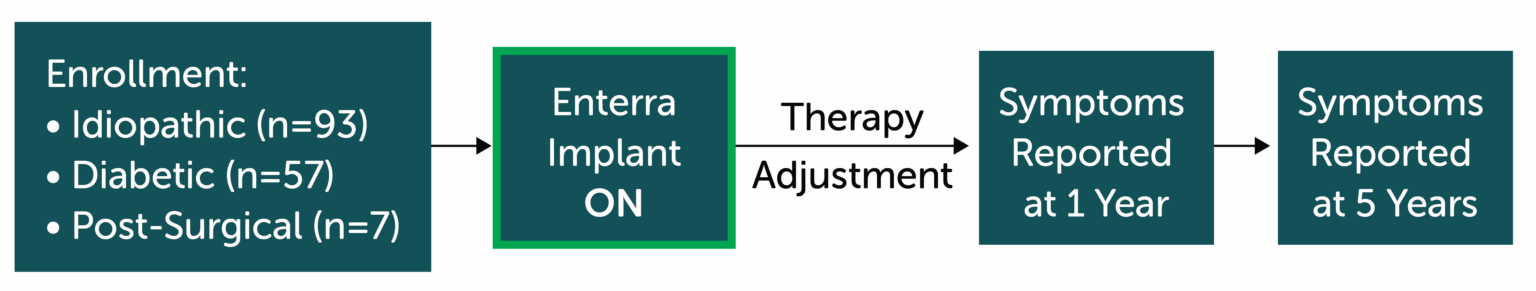
Primary Endpoint
Gastroparesis Cardinal Symptom Index (GCSI) Score at 1 & 5 years vs. Baseline
Results:
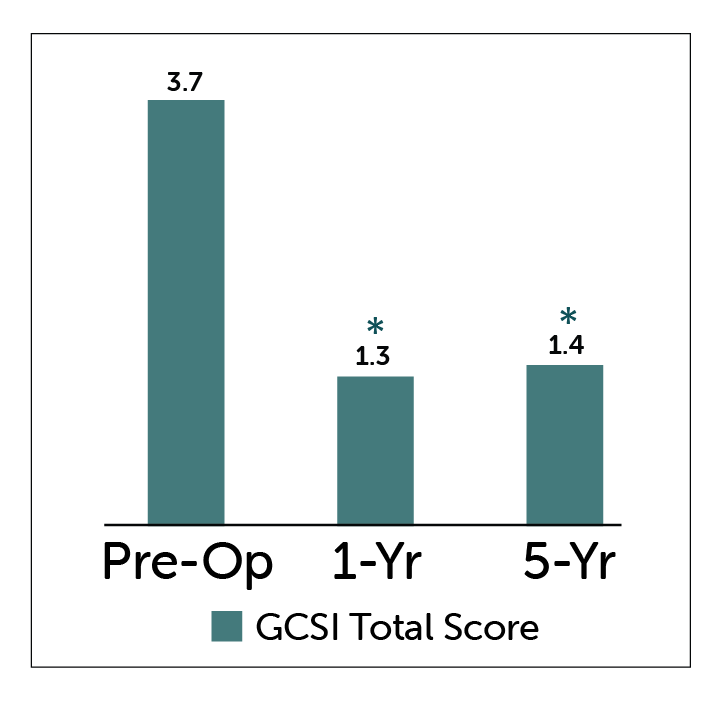
Patients with gastroparesis treated with Enterra Therapy showed statisticaly significant improvements in GCSI Total Score and Nausea-Vomiting Subscores at both 1 and 5 year follow-up.
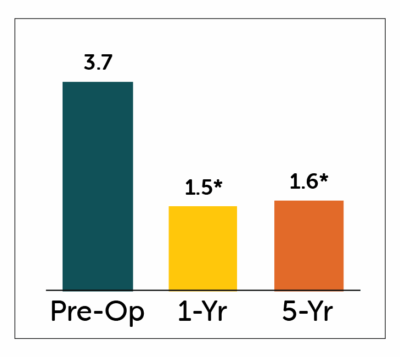
GCSI Total Score
GCSI Subscores
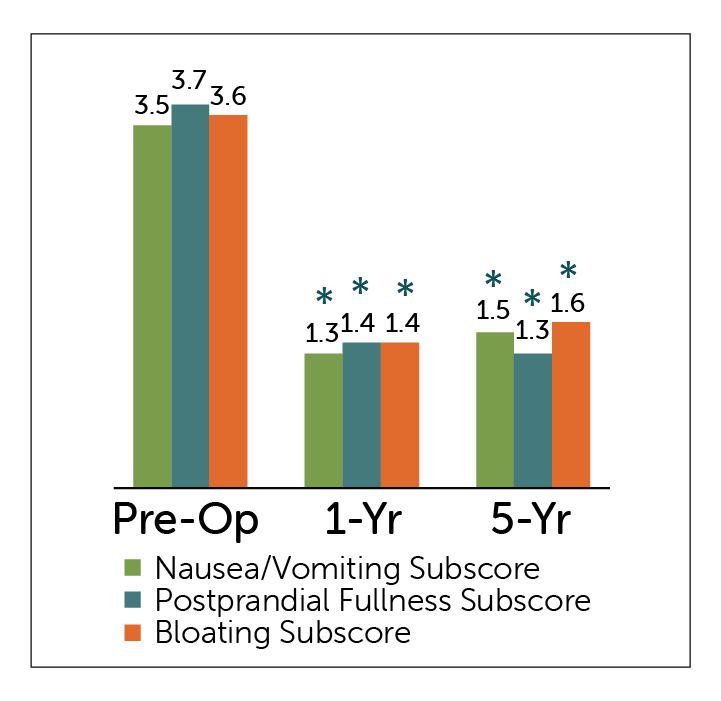
*P<0.05 compared to pre-operative values
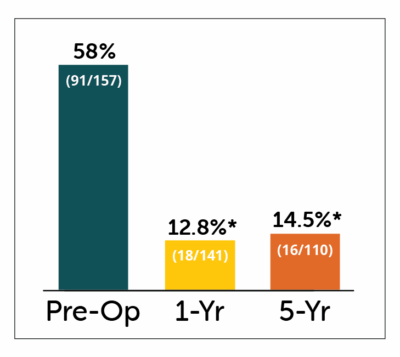
Percent of patients hospitalized, and number of hospitalizations of patients showed significant improvements in patients receiving Enterra Therapy.
% of Patients Hospitalized
for GP in past year
# of Hospitalizations
in past year
*P<0.05 compared to pre-operative values
Patients treated with Enterra Therapy experienced a significant 91% reduction in total number of hospitalization events at 1 year follow-up.
Total Hospitalization Events†
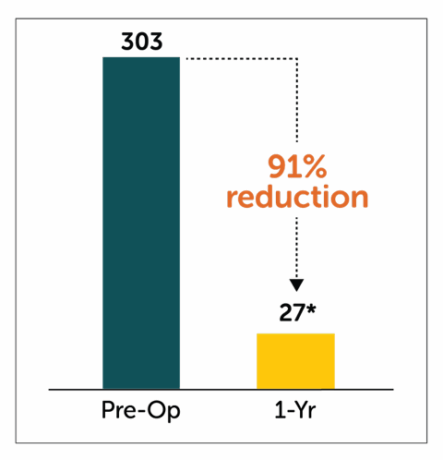
*P<0.05 compared to pre-operative values
†Patient cohort (n=141) with baseline/1-year follow-up data
Adopted from Dr. Michael Awad Podium Presentation. American Foregut Society Annual Meeting. Sept 2024.
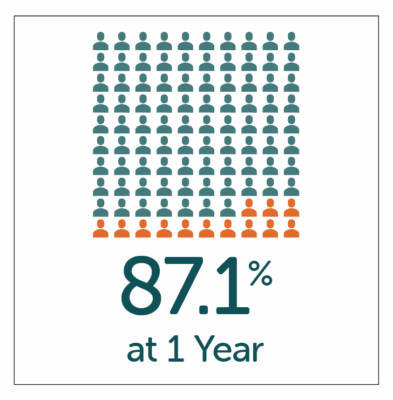
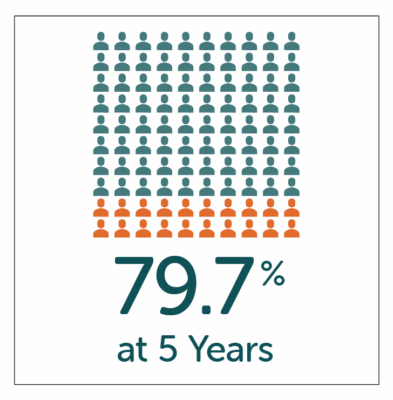
High patient satisfaction with GP symptom relief reported by patients at both 1 and 5 year follow-up after treatment with Enterra Therapy.
Key take-aways:
- In this independently conducted, prospectively collected study of 157 gastroparesis patients examining clinical outcomes of Enterra Therapy, patients receiving Enterra Gastric Electrical Stimulation Therapy showed statistically significant improvements in all Gastroparesis Cardinal Symptom Index (GCSI) scores at 1 year and 5 year follow-up.
- All GCSI Subscales – Nausea/Vomiting; Postprandial Fullness, and Bloating subscales — showed statistically significant improvement at 1 and 5 year follow-up periods.
- Statistically significant improvement in percent of patients hospitalized for gastroparesis & number of hospitalizations at both 1 and 5 year follow-up.
- In 141 patient cohort with both baseline and 1 year follow-up data, total number of hospitalization events showed significant 91% reduction in patients treated with Enterra Therapy.
- High-patient satisfaction of 87.1% and 79.7% reported by patients at both 1 and 5 year follow-up.
- Cassidy DJ, Gerull W, Zike VM, Awad MM. Clinical Outcomes of a Large, Prospective Series of Gastric Electrical Stimulation Patients Using a Multidisciplinary Protocol. J Am Coll Surg. April 2024.
